Induced Pluripotent Stem Cells: A recent discovery that could lead to personalized medicine for SCI

My name is Joe Bonner and I am a postdoctoral fellow in the lab of Dr. Steward at the Reeve-Irvine Research Center and I study the use of human neural stem cells for the treatment of spinal cord injury and my work is currently funded by the California Institute for Regenerative Medicine (CIRM) through a training grant administered at the Bill and Sue Gross Stem Cell Center here at UCI. Stem cells are a special type of cell that have the potential to repair tissue by replacing cells that were lost due to spinal cord injury. Stem cells are present in the brain and spinal cord and they contribute to the normal function of the nervous system throughout life. In the event of a spinal cord injury, resident stem cells respond and make an attempt to repair the damage but there are simply not enough stem cells to fix such serious injuries. In my research, I transplant neural stem cells in an effort to improve tissue repair and to restore the neural connections between the brain and the body. In addition to transplants of stem cells I also administer "growth factors" to improve the function of the transplanted stem cells and at the same time I also manipulate the function of the PTEN/mTOR pathway, which Dr. Steward has previously shown can give the injured nerve cells in the spinal cord a regenerative boost. The goal of my work is to understand how stem cells can contribute to the repair of spinal cord injury and to ultimately create a treatment for people who have suffered a spinal cord injury.
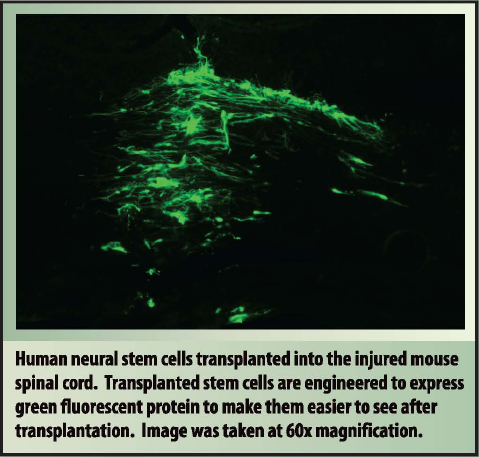
The human neural stem cells that I use in my research are made from a relatively new kind of stem cell called induced pluripotent stem cells, or IPS cells. IPS cells were first described in 2006 by Shinya Yamanaka, who won the Nobel Prize for the discovery in 2012. In the short time IPS cells have been around scientists have begun to understand how they could be applied to treat illness and injury. IPS cells have many characteristics that make them an interesting resource in our search for a treatment for spinal cord injury. IPS cells are made by "reprogramming" or "inducing" an adult cell to become a stem cell. This means that stem cell scientists are able to take a common cell found in an adult, such as a skin cell, and to change that skin cell into a stem cell. This procedure eliminates many of the difficulties that are associated with embryonic stem cells while producing a stem cell with many of the same characteristics. The most intriguing benefit of IPS cells is that they could be made for each individual patient. If an IPS cell therapy was designed to use a patient's own cells then there would be no need for the patient to take additional medications to prevent an immune reaction or transplant rejection.
IPS cells have the potential to make any cell in the adult body, including the cells that make up the spinal cord and brain. IPS cells can be grown for a very long time in a culture dish. This means that cells can be expanded from a small number into enough cells to conduct experiments and to eventually treat human patients. Before IPS cells were discovered, the only type of cell that had these characteristics were embryonic stem cells. Although embryonic stem cells (ESCs) may prove useful for treatment of spinal cord injury, ECS's are not "personalized" so patients who receive ESC transplants have to take immunosuppressant drugs to prevent rejection.
I have been able to demonstrate that human neural stem cells derived from IPS cells can survive in the injured spinal cord and make nerve cells (neurons) and support cells (glia) after transplantation. My continuing research seeks to determine if these transplanted stem cells can communicate with injured nerve cells in the injured spinal cord and if those lines of communication can improve the recovery of function in an animal model of spinal cord injury.





